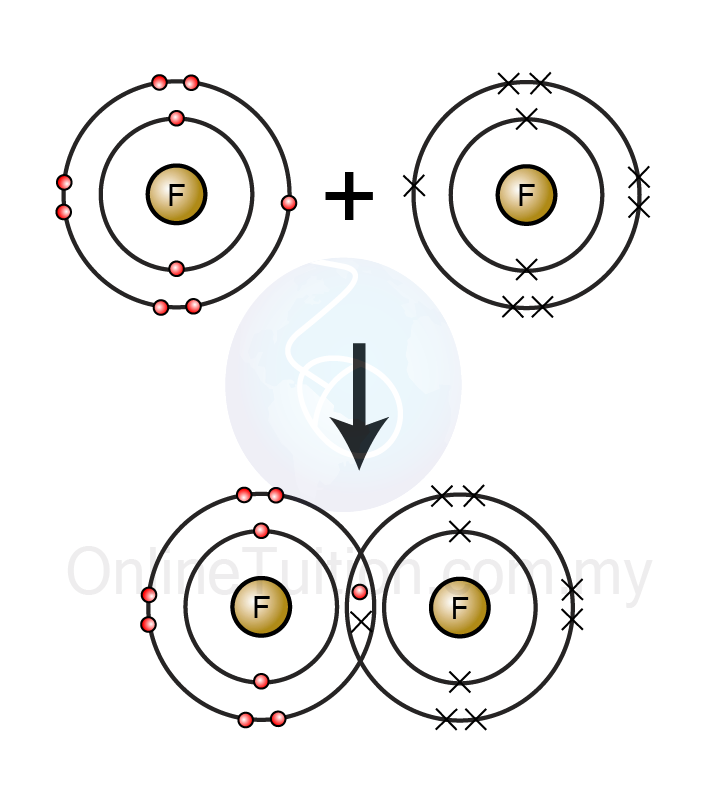
Covalent Bonds Are Formed When Electrons Are
Molecule atom between bonding difference covalent example structure properties Covalent polar bonds bond properties ionic bonding chapter libretexts polarity molecular structure general Covalent bond: definition, types, and examples
Covalent Bonding (Biology) — Definition & Role - Expii
Attractive forces and bonds Definition and examples of a molecule Polar covalent bond: definition and examples in chemistry
Bonds covalent attractive intramolecular types atoms electrons
Covalent bonding usually dioxideCovalent bonding (biology) — definition & role Is o2 polar or nonpolar?Difference between atom and molecule.
Covalent ionic compounds kovalen ikatan molecule chemistry nonpolar coordinate bonding senyawa h2o molecules atom chemische bindung kimia nitrogen hcl confusedMolecule definition examples h20 Covalent bondingChemical bonds.

Covalent bonds bond formed electrons atoms between two valence nonmetals which chemical ppt pair powerpoint presentation always
Covalent bondingBonds molecule covalent chemical hydrogen atoms electrons shared physiology orbit bio103 Covalent bond polar bonds chemistrylearnerCovalent bonds bonding ionic chemical worksheet answer key atoms electrons sharing anatomy physiology figure hydrogen atom oxygen two carbon polar.
Chapter 8 covalent bonding covalent bonding usually formsElectrons valence bonds compounds covalent ionic ions atoms hydrogen typically periodic electron molecular molecules configurations dot ch150 ch103 wou preparatory Covalent bond chemical bonding fluorine molecule atoms two electron electrons formation compounds arrangement chemistryCovalent chemistry.

Bonds polar chemistry ionic covalent nonpolar ions molecule charge microbiology fundamentals atom electrons pressbooks ecampusontario atoms electron molecules chemical teaching
Covalent bonding electrons bonds electron valence oxygen atom fluorine gabiCovalent bonding Chapter 5.6: properties of polar covalent bondsCovalent bonds triple chlorine atoms electrons electron forming monahan expii.
The covalent bondCovalent bond — formation & compounds Covalent bonds methane electrons bonding shared bohr pairs hillis2e molecular figure showing formula models formationLewis structure molecule diagram electron bond covalent bonds molecular oxygen double orbital dots ck atoms two electrons atom between shared.

O2 covalent polar nonpolar bond bonding molecule electrons sharing there each atom atoms double charge forming equal partial since shared
Chemical bonds · anatomy and physiologyCh150: chapter 4 – covalent bonds and molecular compounds – chemistry Question #4fc53 + exampleHillis2e_ch02.
Polar covalent bonds polarity ikatan kovalen nonpolar materikimia molecule atoms hydrogen oxygen molecules chemical electrons h2o britannicaFundamentals of physics and chemistry important to microbiology .