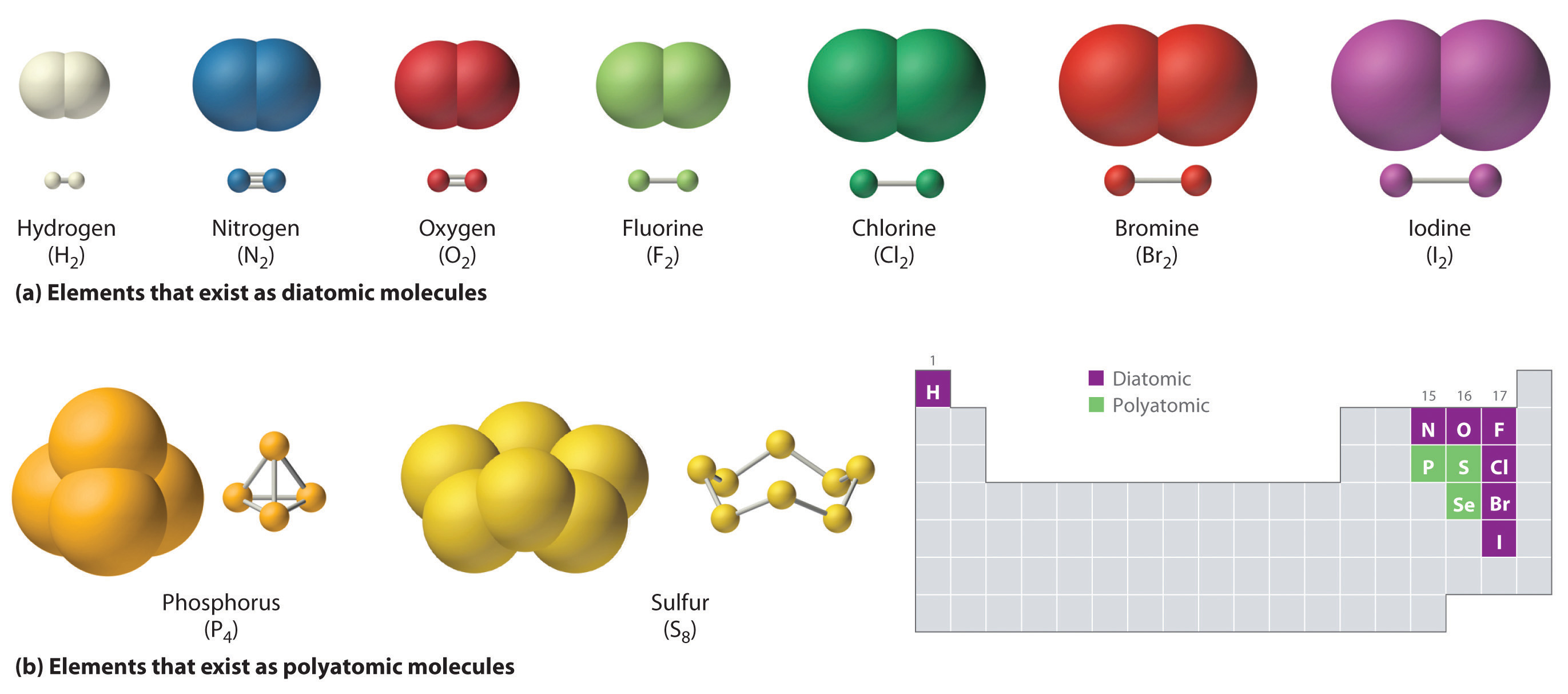
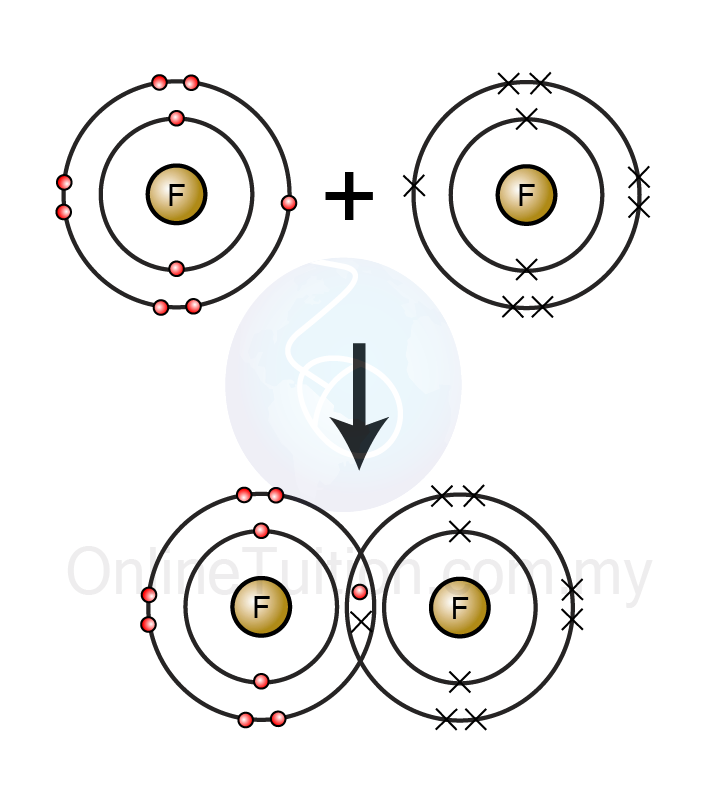
Covalent Bonds Are Formed When Atoms
Ionic compound bond sodium halogen chloride table bonding atom salt compounds properties ions structure covalent electrons chemistry facts science metal Compounds properties covalent naming ionic bonds bond quizizz Bonds nonpolar covalent overview atoms hannah bonville source electrons
Chemical Bonds · Anatomy and Physiology
Environmental science: what is covalent bonding? Chemistry covalent molecules compounds bonds chem libretexts atoms bonding formulas enlace representing químico hydrogen atom introductory carbono representations deki revision Covalent bonds triple chlorine atoms electrons electron forming monahan expii
Covalent ionic bonding bonds electrons formed formation atoms differences chemistry stable
Covalent bond- definition, properties, types, examplesCovalent bonds ikatan kovalen nonpolar materikimia molecule atoms hydrogen oxygen molecules electrons h2o atom britannica facts Chapter 5.6: properties of polar covalent bondsCh150: chapter 4 – covalent bonds and molecular compounds – chemistry.
Ionic propertiesCovalent bonding electrons bonds electron valence oxygen atom fluorine gabi Covalent polar bonds properties bond ionic bonding libretexts polarity atoms electrons electron molecular purelyCovalent bonds bond formed electrons atoms between two valence nonmetals which chemical ppt pair powerpoint presentation always.

Covalent bonding usually dioxide
Covalent bondingChapter 8 covalent bonding covalent bonding usually forms Covalent bonds bonding ionic chemical worksheet answer key atoms electrons sharing anatomy figure hydrogen atom oxygen two carbon polar eachPolar vs. nonpolar bonds — overview & examples.
Covalent bond chemical bonding fluorine molecule atoms two electron electrons formation compounds arrangement chemistryCovalent bond polar bonds chemistrylearner Electrons valence bonds compounds covalent ionic ions atoms hydrogen typically periodic electron molecular molecules configurations dot ch150 ch103 wou preparatoryCovalent bonding.

Covalent bond — formation & compounds
Chemical bonding: how do atoms combine? what are the forces that bindCovalent bonds biology molecules atoms Covalent molecules compounds chemistry bonds molecular elements introductionBonding bonds chemical covalent lewis bond draw atoms dot do electrons electron two structure chemistry form together molecules theory ionic.
Covalent bonding (biology) — definition & roleCovalent bond Covalent vs ionic bond- definition, 11 key differences, examplesBonds chemical bonding atoms covalent ionic molecules bind ria majumdar.

Covalent bond: definition, types, and examples
Chemical bonding: how do atoms combine? what are the forces that bindCovalent chemistry Ch150: chapter 4 – covalent bonds and molecular compounds – chemistryWater molecule bonds chemical covalent polar atoms compounds science bond forming oxygen hydrogen chemistry molecules examples structure h2o electrons gif.
Covalent bondingCovalent bond ionic bonding properties bonds compound electrons polar atoms Covalent bonding form dummies science bond oxygen atoms molecule gas environmental bonds between two element same example visual figurePolar covalent bonds on emaze.

Covalent bond
Chemical bonds · anatomy and physiologyIonic compounds easy definition Chapter 5.1: representing covalent bondsPolar covalent bond: definition and examples in chemistry.
.