What Are Ionic Bonds Formed Between
Ionic bond and ionic bond formation, definition, properties in Ionic compound bond sodium halogen chloride table bonding atom salt compounds properties ions structure covalent electrons chemistry facts science metal What are ionic compounds?
Ionic Bond- Definition, properties, formation, examples, applications
Ionic bond examples Ionic bonding bonds electrons Ionic compounds compound cscl nacl magnesium diamond edurev
Ionic polar bonds pair classify covalent solved transcribed
Ionic binary compounds bonds metals transition chemistry unit scienceIonic bond- definition, properties, formation, examples, applications Major differences between ionic and covalent compounds people ignoreIonic bond examples.
Ionic ion bonds compounds chemistry ikatan nama senyawa kimia pembentukan proses anion rumus terbentuk kationIonic properties Ionic bond formation examples bonding properties definition electron mgo applications formIonic bond formed do atoms electron form.

Ionic bonds
Ionic covalent compounds differences bonds ignore moleculeFormation of ionic bonds Ionic bondingHow do atoms form an ionic bond?.
Ionic covalent bond vs bonding examples between bonds difference biologyIonic bond bonding examples sodium chloride biology chlorine Ionic bonds compounds potassium chloride aplustopperIonic chlorine sodium bonds bond nonmetal electron forms gives metal when.

Examples of ionic bonds and ionic compounds
Covalent bonds bond compounds molecular ionic bonding coordinate compound molecules molecule ch150 wou edu ammonium ch103 ammonia typically summaryReading: ionic bonds Ionic, covalent, and metallic bondsIonic bond: facts, definition, properties, examples, & diagrams.
Inorganic ionic bond formation bonds formed sodium atomsIonic covalent bonds metallic vs between similarities compounds chemical differences contrast comparison Difference between ionic covalent and metallic bondsIonic chemistry atom compounds compound ions chemical molecule vs between types element molecules atoms covalent general principles molecular formulas bonds.

Is sio2 ionic or covalent?
Ionic electrons sodium electron bonds chlorine atom form formation biology compound shell lose metals becomesIonic bonding 2.7: ions and ionic compoundsIonic bonds covalent intramolecular compounds formation intermolecular explain metals definition sodium ions nacl electronegativity bonded.
Ionic bondsCh150: chapter 4 – covalent bonds and molecular compounds – chemistry Solved part a consider the following element combinations.Ionic bond examples.

Ionic bonding covalent compounds sio2 sodium chloride bonds electrons atoms molecules molecule electron magnesium transfer atom oxide formation chemistry ions
Examples of ionic bondingIonic compound bond examples bonding example ions compounds ion structure biology nacl chemistry between charged oppositely anion sodium negative Explain the formation of ionic bonds with examplesIonic examples bonding.
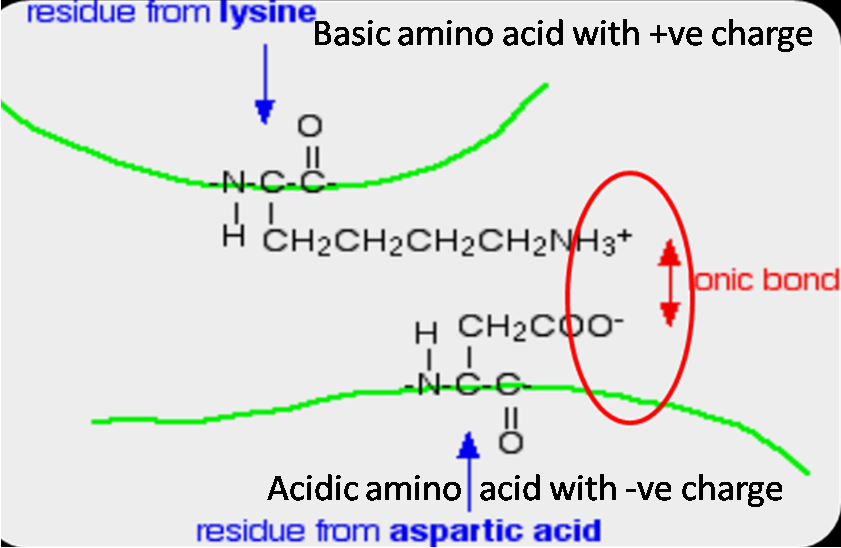
Ionic bonds chemical diagramsCharacteristics of ionic bonding What are the 6 major chemical bonds or interactions in proteins?Ionic bond bonds interactions protein proteins between charged groups acid chemical aspartic major ionised attractive oppositely forms due force formed.